 Authors: Jennifer Harbottle (RINH), Surita Lawes (Loreto), Sanjai Patel (FBMH), Andreas Prokop (FBMH)
Authors: Jennifer Harbottle (RINH), Surita Lawes (Loreto), Sanjai Patel (FBMH), Andreas Prokop (FBMH)
Get this lesson & adjunct materials:
 L2-KS5-Genes&Alcohol.zip [LINK]
L2-KS5-Genes&Alcohol.zip [LINK] L2-Spanish-Genes&Alcohol.zip [LINK]
L2-Spanish-Genes&Alcohol.zip [LINK] L2-Turkish-ALKOL_DERSI.zip [LINK]
L2-Turkish-ALKOL_DERSI.zip [LINK] Indonesian translation of website [LINK]
Indonesian translation of website [LINK]
Curriculum relevance: metabolism and biological reactions, the genetic code, protein synthesis, genetic diversity, natural selection
Summary: This lesson is a synoptic resource suitable for Biology A Level (KS5), ideal for end-of-term revision lessons. It uses the theme of alcohol and alcohol tolerance to conceptually link and review the topics of fermentation, metabolism, enzymes, the genetic code and protein synthesis, genetic/allelic diversity and adaptation, natural selection and evolution, and even pharmacological concepts combined with classical conditioning. Experiments and activities include a chemical reaction that demonstrates enzymatic activity in live tissues of normal versus enzyme-deficient maggots, a behavioural experiment demonstrating alcohol tolerance, and an activity on genetic code, transcription and translation.
Support information for this lesson:
- Where does alcohol come from? -> GO
- Alcohol and human culture -> GO
- The breakdown of alcohol requires enzymes encoded by genes -> GO
- What happens if alcohol cannot be broken down? -> GO
- Treating alcohol abuse with drugs -> GO
- Natural variation in alcohol tolerance -> GO
- Also ADH and ALDH of flies display natural variations -> GO
- Adh alleles undergo Darwinian selection processes -> GO
- A global geographical distribution of Adh alleles -> GO
- Global patterns of ADH and ALDH alleles in humans -> GO
1. Where does alcohol come from?
Yeasts such as Saccharomyces cerevisiae, and other members of the same genus, break down sugars through the process of alcohol fermentation which produces alcohol (ethanol) and yields two molecules of ATP per glucose molecule. ATP is the essential energy source of living cells! The ATP is produced during a process called glycolysis breaking the sugar up into two pyruvates; glycolysis requires NAD+, which is regenerated in the fermentation step when pyruvate is broken down into alcohol (see image).
Other forms of ATP production
To generate ATP efficiently, most cells undergo aerobic respiration (net formula starting from glucose: C6H1206 + 6 O2 → 6 H2O + 6 CO2 + energy): they import the intermediate break-down product pyruvate into their mitochondria where it is further broken down into CO2, first via the pyruvate dehydrogenase (PDH) reaction followed by the Krebs/citrate cycle. In this process, pyruvate or other organic molecules serve as electron donators, releasing electrons that are shuttled via NADH and FADH2 to the oxidative phosphorylation/electron transport pathway. This pathway involves a number of protein complexes that make up the electron transport chain (dark green in inset). This chain uses the high energy electrons shuttled in by NADH and FADH2 and passes them step-wise down (yellow stippled line in inset) to oxygen as the ultimate electron acceptor, thus generating water. This process generates energy which is used to export H+ into the intermembrane space (light blue). In consequence, an electro-chemical gradient forms across the inner mitochondrial membrane where H+ wants to flow back into the mitochondrial lumen. Vaguely comparable to water wheels, so-called ATPases (red in inset) use the H+ influx to generate ATP, with a net outcome of ~34 ATP from one glucose molecule in addition to the 2 ATP generated during glycolysis.
When starved of oxygen, cells usually undergo anaerobic fermentation, which turns pyruvate into lactate (lactic acid) as end product and, like alcohol fermentation, regenerates NAD+ without producing any further ATPs. Note that our red blood cells always depend on fermentation because they contain no mitochondria.
Some organisms are adapted to oxygen-deprived environments. They generate ATP via anaerobic respiration (see also here) by using less powerful molecules than oxygen as electron acceptors; organic or even inorganic molecules (e.g. hydrogen gas) serve as electron donators. For example, bacteria in the soil of coast wetlands or at volcanic/hydothermal vents perform sulfate respiration: they ‘breath’ sulfate (SO42−) as electron acceptor, turning it into hydrogen sulfide (H2S) with a characteristic ‘rotten egg’ smell. The generated ATP and H2S can be used for chemosynthesis to generate carbohydrates (12 H2S + 6 CO2 → C6H12O6 + 6 H2O + 12 S) – comparable to plants where ATP (generated by photosynthesis instead of respiration) drives the Calvin cycle to bind CO2 into higher order molecules. Anaerobic respiration can have large impact on our environment. For example, other forms of anaerobic respiration produce methane, as a major component of our farts and acting as an extremely powerful greenhouse gas that contributes to global warming (especially when bacterial methane stored in frozen soils and lakes of the tundra are released due to ongoing thawing).
Like aerobic respiration in mitochondria, also anaerobic respiration of bacteria uses an electron transport chain to pump H+ into the outer space, and ATPases driven by H+ flux to generate ATP. This is why it is generally believed that mitochondria originate from bacteria which were incorporated into Eukaryote cells to form a symbiontic relationship (endosymbiontic theory).
Fermenting yeasts usually live in aerobic conditions, but they still choose not to capitalise on the energy-efficient option of aerobic respiration, although they are equipped with mitochondria. They are therefore classified as facultative (=optional) anaerobes. One theory is that yeasts use fermentation to create their own environmental niche. Thus, alcohol is deadly for most other organisms, whereas fermenting yeasts are unusually resistant to relatively high alcohol concentrations. By performing energy-inefficient, alcohol-producing fermentation, they fabricate therefore a niche in which they have a competitive advantage. This is why yeasts are being used to produce wine and beer, but this also means that many rotting fruits out in nature tend to be rich in alcohol.
2. Alcohol and human culture
 Our ancestors have been producing and consuming alcohol since the Neolithic era, and the widespread production and consumption of wine, beer and spirits has gained a significant social role in many cultures. In those cultures, many adults tend to enjoy the occasional social alcoholic drink, its taste and relaxing effects, for example on a typical night out at the weekend. But alcohol is present in our lives more often than we think. Many day-to-day foods contain trace amounts of alcohol, including fruit, freshly baked bread and fermented dairy products. Alcohol is also found in mouth wash, cosmetics and cough medicines, and our own body produces alcohol during digestion.
Our ancestors have been producing and consuming alcohol since the Neolithic era, and the widespread production and consumption of wine, beer and spirits has gained a significant social role in many cultures. In those cultures, many adults tend to enjoy the occasional social alcoholic drink, its taste and relaxing effects, for example on a typical night out at the weekend. But alcohol is present in our lives more often than we think. Many day-to-day foods contain trace amounts of alcohol, including fruit, freshly baked bread and fermented dairy products. Alcohol is also found in mouth wash, cosmetics and cough medicines, and our own body produces alcohol during digestion.
 Modest alcohol consumption can be in agreement with a sensible and healthy lifestyle. However, some people are not able to control the amount of alcohol they drink, and consume a lot more than the recommended upper limit, and risk levels are explained on this website. Alcohol is a commonly abused psychoactive drug that can result in short-term, acute behavioural problems (violence, drink driving, unprotected sex) or in chronic addiction. Although alcohol addiction is a complex interplay of environmental and social factors, it often runs in families, and genes are thought to be responsible for 50% of the risk of developing alcoholism. This may indicate a partial genetic basis for alcohol-related problems. Systematic research is therefore being carried out to identify such potential genetic factors, aiming to develop cures that have a greater chance of success.
Modest alcohol consumption can be in agreement with a sensible and healthy lifestyle. However, some people are not able to control the amount of alcohol they drink, and consume a lot more than the recommended upper limit, and risk levels are explained on this website. Alcohol is a commonly abused psychoactive drug that can result in short-term, acute behavioural problems (violence, drink driving, unprotected sex) or in chronic addiction. Although alcohol addiction is a complex interplay of environmental and social factors, it often runs in families, and genes are thought to be responsible for 50% of the risk of developing alcoholism. This may indicate a partial genetic basis for alcohol-related problems. Systematic research is therefore being carried out to identify such potential genetic factors, aiming to develop cures that have a greater chance of success.
Even animals can be tempted
3. The breakdown of alcohol requires enzymes encoded by genes
Alcohol is not unique to human culture, but similarly present in nature, and rotting fruit can contain over 4% alcohol. Wild animals, such as birds and squirrels have often been documented to seek out and eat this fermented fruit and feel the effects of slight intoxication (LINK1; LINK2). To protect animals from alcohol intoxication, all organisms have evolved biological means to decompose and remove it from our bodies, a process generally referred to as catabolism. Without this ability, alcohol would build up in our bodies and we would be in a constant state of intoxication and eventually die of alcohol poisoning.
Usually, chemical substances are produced or broken down in cells via step-wise processes referred to as metabolic pathways, in which a series of specialised proteins (enzymes; see next section) introduces alterations to the chemical substance that generates an intermediate product used as substrate by the next enzyme in the pathway. Metabolism can be divided into two categories: catabolism (the breakdown of molecules to obtain energy or remove potential toxic effects) and anabolism (the synthesis of compounds usually requiring energy).
Enzymes are proteins that act as biological catalysts and drive chemical reactions forward by lowering their activation energy. Enzymes possess an active site to which a complementary molecule, known as the substrate, can bind and form an enzyme-substrate complex. When this specific substrate fits into the active site, the whole enzyme changes its conformation to accommodate and hold the substrate in exactly the right position for chemical reactions to occur. This is known as the model of induced fit. Most enzymes also require co-enzymes, which are non-protein compounds that assist in catalysis of the chemical reaction. An inactive enzyme without a co-enzyme is called an apoenzyme, and a complete enzyme with co-enzyme that is ready for substrate binding, is called holoenzyme. The substrate is modified during the reaction and once complete, the product(s) leave(s) the active site and the enzyme becomes available to bind another substrate molecule.

Break-down of alcohol (ethanol) into acetic acic as an example for a metabolic/catabolic pathway: ADH and ALDH both possess two binding sites, one where the primary substrate (ethanol and acetaldehyde, respectively) binds, and one where the co-enzyme NAD+ binds.
The catalytic pathway breaking down alcohol (see image above and animation below) is carried out in a two-step process mediated by by two specialised  enzymes, alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH), each encoded by a different genes. (1) In the first step, ADH in the cytoplasm of liver or stomach cells catalyses the conversion of ethanol to acetaldehyde. Acetaldehyde is a highly toxic substance and known carcinogen. It has a powerful vasodilating effects (= it widens blood vessels) and causes the face to become red (alcohol flush reaction). It is also the substance that is responsible for unpleasant physiological effects associated with a hangover (nausea, headache, heart palpitations). (2) The second enzyme ALDH, removes acetaldehyde by catalysing its conversion into acetic acid, which can then be broken down into CO2 and water via the Krebs cycle.
enzymes, alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH), each encoded by a different genes. (1) In the first step, ADH in the cytoplasm of liver or stomach cells catalyses the conversion of ethanol to acetaldehyde. Acetaldehyde is a highly toxic substance and known carcinogen. It has a powerful vasodilating effects (= it widens blood vessels) and causes the face to become red (alcohol flush reaction). It is also the substance that is responsible for unpleasant physiological effects associated with a hangover (nausea, headache, heart palpitations). (2) The second enzyme ALDH, removes acetaldehyde by catalysing its conversion into acetic acid, which can then be broken down into CO2 and water via the Krebs cycle.

Enzymes are proteins that catalyse chemical reactions and are encoded by genes, here illustrated with the breakdown of alcohol (ethanol). Follow one green block from left to right through the whole process. Click image to enlarge.
Note that acetic acid is the key molecule in vinegar and that vinegar is often produced from alcohol, e.g. wine. In this case, acetic acid bacteria (usually airborne) use a different reaction using oxidation of alcohol with O2.
4. What happens if alcohol cannot be broken down?
 Fruit flies have an almost daily need for alcohol detoxification, since rotting fruits, and yeasts within, are the preferred niche where they feed and lay their eggs. Accordingly, they carry equivalents of the ADH and ALDH enzymes, which have been intensely studied capitalising on the ease of genetic research in flies (see the “Why Fly?” tab).The importance of ADH and ALDH during alcohol metabolism is best illustrated by mutant flies that lack the activity of one of these enzymes, a condition which makes them highly sensitive to the toxic effects of alcohol compared with normal (= wildtype or WT) flies. Thus, flies that carry mutations in either the Adh or the Aldh gene and in a way that both existing copies of this gene are affected (homozygosis), do not survive exposure to ethanol levels above 4% – which is the typical concentration you would find in fermented fruit or beer. As illustrated in the film below, these mutant flies show high mortality rates at alcohol concentrations that do not harm wildtype flies with normal enzyme activity.
Fruit flies have an almost daily need for alcohol detoxification, since rotting fruits, and yeasts within, are the preferred niche where they feed and lay their eggs. Accordingly, they carry equivalents of the ADH and ALDH enzymes, which have been intensely studied capitalising on the ease of genetic research in flies (see the “Why Fly?” tab).The importance of ADH and ALDH during alcohol metabolism is best illustrated by mutant flies that lack the activity of one of these enzymes, a condition which makes them highly sensitive to the toxic effects of alcohol compared with normal (= wildtype or WT) flies. Thus, flies that carry mutations in either the Adh or the Aldh gene and in a way that both existing copies of this gene are affected (homozygosis), do not survive exposure to ethanol levels above 4% – which is the typical concentration you would find in fermented fruit or beer. As illustrated in the film below, these mutant flies show high mortality rates at alcohol concentrations that do not harm wildtype flies with normal enzyme activity.
When using our knowledge of the metabolic pathway for alcohol break-down (described above), it becomes clear what happens upon consumption of alcohol: we build up toxic substances, as illustrated by the following animation:

In this animation, the gene for the ALDH gene carries a mutation rendering the enzyme non-functional. In consequence, highly toxic acetaldehyde builds up.

Upon ADH deficiency, toxic alcohol accumulates, explaining the death of flies upon alcohol inhalation (see film above). Note, that also production of the NADH by-product is blocked, which is used for the staining reaction explained below.
The activity of enzymes can often be made visible. For example, the activity of the ADH enzyme can be made visible with a simple colour reaction that takes only 5 minutes and can be easily carried out in the class room:

A simple experiment demonstrating the genetics of Adh enzyme activity. Larvae are easily dissected (left). The time lapse sequence shows a 5 minute colour reaction indicating ADH enzyme activity in the normal maggot; in contrast, the Adh mutant maggot shows no colour reaction, hence lacks ADH activity.
The colour reaction is explained here: when ADH catalyses the break-down of 2-butanol into butanone, it generates NADH. In the presence of phenazine methosulfate (PMS), NADH drives the reduction of nitro blue tetrazolium (NBT) into the violet colour formazan.
Fruit flies display “human-like” alcohol-related behaviours
 You may be surprised to hear that the fruit fly Drosophila melanogaster is being used to study potential genetic and biological mechanisms that underlie alcohol metabolism as well as alcohol abuse and addiction. As is explained under the “Why Fly?” tab, Drosophila offers efficient strategies for research i
You may be surprised to hear that the fruit fly Drosophila melanogaster is being used to study potential genetic and biological mechanisms that underlie alcohol metabolism as well as alcohol abuse and addiction. As is explained under the “Why Fly?” tab, Drosophila offers efficient strategies for research i n many areas of biology, and this often has helped to understand similar processes in higher animals and humans. Accordingly, there is a realistic chance that also fly research into alcohol-related biology may contribute towards the development of more efficient and effective therapies in humans, as is explained in greater detail in this blog post. To illustrate how far comparisons to humans can go, just see whether some of the following facts sounds familiar to you (see also a collection of videos):
n many areas of biology, and this often has helped to understand similar processes in higher animals and humans. Accordingly, there is a realistic chance that also fly research into alcohol-related biology may contribute towards the development of more efficient and effective therapies in humans, as is explained in greater detail in this blog post. To illustrate how far comparisons to humans can go, just see whether some of the following facts sounds familiar to you (see also a collection of videos):
- Flies become hyperactive and tipsy, and eventually pass out. Initially, alcohol has a stimulant effect and flies become hyperactive; this is similar to increased energy levels and decreased inhibition in humans. As alcohol concentration increases, flies begin to lose motor coordination and soon fall over, bump into each other and struggle to climb walls. At higher doses of alcohol, the rewarding and “tipsy” phase disappears, and the alcohol has a sedative effect (see accompanying film “KS5-Genes&Alcohol-DrunkFly.wmv”).
-
Drunk male flies become hypersexual and promiscuous; for example, they start courting other males – They take the “beer goggle” effect to a whole new level!
- Male flies that have been sexually rejected turn to alcohol and drink more as a coping strategy! (see this video)
- Flies show signs of alcohol addiction and seek out the drug despite adverse consequences. They will overcome the bitter and aversive taste of quinine, and even face increasingly powerful electric shocks to drink alcohol!
- Flies experience withdrawal symptoms (increased susceptibility to seizures) and relapse-like behaviour.
Do you agree that Drosophila displays fundamental characteristics which you would have thought to be specific human behaviours?
5. Treating alcohol abuse with drugs
 The development of alcohol abuse disorders results in serious health and interpersonal relationship problems, and affects society as a whole by putting a strain on medical and economic resources. Attempts have been made to treat alcohol addiction pharmacologically, using ADH and ALDH as the drug targets. To achieve this, drug designers make use of the fact that substrates are specific to an enzyme’s active site. They aim to design drugs that mimic the substrate’s ability to bind the enzyme’s active site but cannot be metabolised. In consequence, these drugs block the active site and prevent the formation of the natural enzyme-substrate complex. Such a molecule or drug is called an enzymatic inhibitor.
The development of alcohol abuse disorders results in serious health and interpersonal relationship problems, and affects society as a whole by putting a strain on medical and economic resources. Attempts have been made to treat alcohol addiction pharmacologically, using ADH and ALDH as the drug targets. To achieve this, drug designers make use of the fact that substrates are specific to an enzyme’s active site. They aim to design drugs that mimic the substrate’s ability to bind the enzyme’s active site but cannot be metabolised. In consequence, these drugs block the active site and prevent the formation of the natural enzyme-substrate complex. Such a molecule or drug is called an enzymatic inhibitor.
 One such inhibitor is disulfiram which is commercially known as Antabuse® and has been used to support abstinence in the treatment of alcohol abuse since 1948. Disulfiram is broken down by our body and the metabolic products generated through this process, covalently bind to the active site and irreversibly inhibit ALDH activity. As a result, acetaldehyde cannot be converted to acetic acid and builds up in the body. Therefore, when taking disulfiram during alcohol consumption, this triggers the immediate and violent set of physiological symptoms caused by high levels of acetaldehyde (see section 3). The outcome is learned behaviour referred to as classical conditioning, where alcohol is associated with bad memories and therefore avoided in future. This is an aversive strategy to deter alcoholics from drinking during the withdrawal phase of treatment.
One such inhibitor is disulfiram which is commercially known as Antabuse® and has been used to support abstinence in the treatment of alcohol abuse since 1948. Disulfiram is broken down by our body and the metabolic products generated through this process, covalently bind to the active site and irreversibly inhibit ALDH activity. As a result, acetaldehyde cannot be converted to acetic acid and builds up in the body. Therefore, when taking disulfiram during alcohol consumption, this triggers the immediate and violent set of physiological symptoms caused by high levels of acetaldehyde (see section 3). The outcome is learned behaviour referred to as classical conditioning, where alcohol is associated with bad memories and therefore avoided in future. This is an aversive strategy to deter alcoholics from drinking during the withdrawal phase of treatment.
Disulfiram therefore modulates alcohol sensitivity by inhibiting an essential enzyme in alcohol metabolism. This example of a pharmacological competitive inhibitor demonstrates the importance of understanding and targeting fundamental biological reactions in order to make advances in therapeutics.
6. Natural variation in alcohol tolerance
We have seen that alcohol tolerance involves active detoxification through a two-step break-down process catalysed by the ADH and ALDH enzymes.
However, as for everything in biology, there is natural variation in this enzymatic pathway. Due to genetic variation, ADH and/or ALDH may show altered levels of activity. In consequence, some people are less tolerant to alcohol levels than others:
- ADH activity may be low, so that ethanol circulates in the bloodstream for longer (prolonged state of intoxication). For example, women have less ADH enzyme in the stomach than men do. Therefore, if a woman and a man of the same weight drink the same amount of alcohol under the same circumstances, women tend to have a higher blood alcohol concentration than the man because the alcohol they consume takes longer to be broken down. This explains why women are usually more sensitive to a round of drinks than men.
- ADH activity may be moderately increased, so that there is an increased rate of conversion to acetaldehyde which then triggers a higher conversion into acetic acid, thus leading to efficient detoxification. An example is the Drosophila ADH-F variant (see section 9).
- ADH activity may be excessively high, as is the case for the human ADH1B*2 allele/variant (see section 11). This variant has an alcohol turn-over rate 40 times faster than the typical ADH1B*1 allele variant. Due to these unusually high turn-over rates, acetaldehyde therefore builds up and results in unpleasant side effects.
- ALDH activity may be low, causing a high sensitivity to alcohol (low tolerance) due to a build-up of acetaldehyde – similar to the previous example but through a different mechanism. An example is the human ALDH2*2 variant (see section 11) which has a lower enzymatic turn-over. This allele is, in a way, a genetic version of the same effect as produced pharmacologically when using the disulfiram drug (see section 5).
- ALDH activity may be high, so that there is an increased rate of acetaldehyde clearance which promotes a high turn-over of alcohol and high alcohol tolerance.
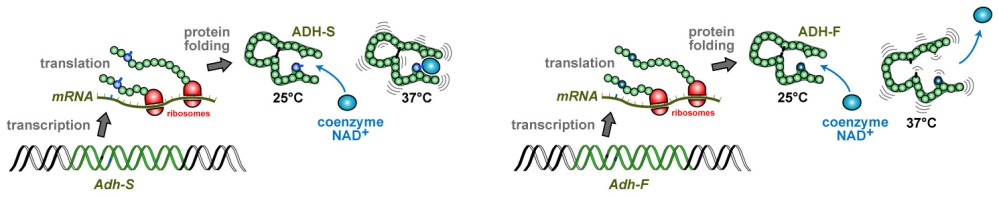
7. Also ADH and ALDH of flies display natural variations
 Mutant flies without enzyme activity have an important disadvantage and are rare in nature. However, natural populations of Drosophila melanogaster across the world are polymorphic for two abundant alleles of the Adh gene: Adh-S and Adh-F. These alleles code for variants of the same protein which differ by a single amino acid in the same specific position: Adh-F contains a positively charged lysine and Adh-S an uncharged threonine. Electrophoresis techniques can be used to separate proteins from one another, as a function of their size and charge. In such experiments, ADH-S behaves “slow” whereas ADH-F runs “fast”, hence the allele names “S” and “F”. But what is the functional relevance of these alleles?
Mutant flies without enzyme activity have an important disadvantage and are rare in nature. However, natural populations of Drosophila melanogaster across the world are polymorphic for two abundant alleles of the Adh gene: Adh-S and Adh-F. These alleles code for variants of the same protein which differ by a single amino acid in the same specific position: Adh-F contains a positively charged lysine and Adh-S an uncharged threonine. Electrophoresis techniques can be used to separate proteins from one another, as a function of their size and charge. In such experiments, ADH-S behaves “slow” whereas ADH-F runs “fast”, hence the allele names “S” and “F”. But what is the functional relevance of these alleles?
8. Adh alleles undergo Darwinian selection processes
 It was found that Adh-F flies have a 2.5- to 3-fold higher ADH enzyme activity compared with Adh-S flies, and animals carrying one copy of Adh-F together with a copy of Adh-S (referred to as heteroallelic constellation) have an intermediate level of activity.
It was found that Adh-F flies have a 2.5- to 3-fold higher ADH enzyme activity compared with Adh-S flies, and animals carrying one copy of Adh-F together with a copy of Adh-S (referred to as heteroallelic constellation) have an intermediate level of activity.
But how does a single amino acid change cause a difference in ADH enzyme activity? Two reasons were identified:
- The Adh-F allele causes more ADH protein to be produced, and the presence of more enzyme means a higher alcohol turn-over rate.
- The ADH-F enzyme binds to its NAD+ coenzyme less strongly than does ADH-S. In consequence, dissociation of NAD+ from the ADH-F enzyme-substrate complex is faster so that more alcohol can be turned over per unit of time as compared to ADH-S.
9. A global geographical distribution of Adh alleles
 From the above example, you may ask why the Adh-F allele, which is so efficient in protecting against alcohol intoxication, does not take over in all fly populations? Obviously, the Adh-S allele seems to have something on offer which is of advantage under different conditions – important enough that it is kept long-term in genetic pools. What may this trait of ADH-S be?
From the above example, you may ask why the Adh-F allele, which is so efficient in protecting against alcohol intoxication, does not take over in all fly populations? Obviously, the Adh-S allele seems to have something on offer which is of advantage under different conditions – important enough that it is kept long-term in genetic pools. What may this trait of ADH-S be?
Interesting hints came from the study of allele distributions across the world which revealed a global pattern: the Adh-S allele shows a higher frequency in equatorial regions including Africa, whereas Adh-F predominates in temperate regions. This observation led to the hypothesis that higher temperatures in equatorial regions might be a selection parameter, and this hypothesis was tested in the laboratory: when scientists maintained genetically varied fly populations for several generations at different temperatures, they found that the abundance of the Adh-S allele increased at a high temperature (29.5°C), but dropped at lower temperatures (20°C and 25°C). These findings are in agreement with the hypothesis that ADH-S has a selection advantage at higher temperatures. But through which mechanisms might temperature promote the abundance of Adh-S?
One theory relates to ADH enzyme stability. With increasing temperature, an enzyme molecule vibrates more energetically, and the protein’s architecture needs to be stable to prevent unfolding accompanied by inactivation. The ADH-S isoenzyme binds its coenzyme NAD+ more strongly than does ADH-F, thereby conferring greater stability to the holoenzyme. It is therefore more resistant to increases in environmental temperature. At higher temperatures, it seems therefore of advantage to play safe and guarantee basal levels of enzyme activity provided by ADH-S, even if its alcohol turn-over rate is lower than that of ADH-F.
10. Global patterns of ADH and ALDH alleles in humans

 Also allelic variants of human ADH and ALDH show a global geographical pattern. However, these patterns are different from those found for Drosophila. They do not display a north-south but a west-east divide: people of eastern Asian descent have a higher likelihood to carry ADH or ALDH variants that render them more sensitive to alcohol, whereas Caucasians are usually more tolerant.
Also allelic variants of human ADH and ALDH show a global geographical pattern. However, these patterns are different from those found for Drosophila. They do not display a north-south but a west-east divide: people of eastern Asian descent have a higher likelihood to carry ADH or ALDH variants that render them more sensitive to alcohol, whereas Caucasians are usually more tolerant.
What may the causes for this distribution be? We learned that temperature is a likely selection criterion for Adh-S and Adh-F in flies. But this criterion is less likely to apply to humans since humans are endotherm (warm-blooded; Greek: endos = within, thermos = hot), hence less dependent on temperature change than flies which are ectotherm (cold-blooded; ektos = outside). People of Asian descent frequently carry the ADH1B*2 and/or the ALDH2*2 alleles which cause excessive build-up of acetaldehyde upon alcohol consumption (see section 6):
- ADH1B*2 carries a point mutation which causes an amino acid substitution (histidine to arginine) leading to 40 times faster conversion of ethanol to acetaldehyde.
- ALDH2*2: This amino acid substitution (Glutamine to Lysine) causes a weaker NAD+ binding site leading to lower enzymatic turn-over. ALDH2*2 therefore causes acetaldehyde to build up in the blood following alcohol consumption.
The ADH1B*2 and ALDH2*2 variants both act in a dominant manner. Therefore, people who carry one (heterozygote) or two (homozygote) copies of either allele are prone to high acetaldehyde levels and its toxic, violently negative physiological effects (see section 3). As a result, carriers of these genetic variants are less likely to drink alcohol and are at less risk of alcohol abuse disorders, consistent with the idea that genetic or drug-induced build-up of acetaldehyde is protective against alcohol dependence (see section 5). But why are these allelic variants more frequent in Asian populations?
- One hypothesis is that ADH and ALDH may have of that these alleles have other advantages, as similarly described for Drosophila ADH-S (see section 10), but likely in the context of other biological functions beyond alcohol metabolism. What the selective parameters might be is not known, but these functions may be (or may evolutionary have been) more essential than the risk of toxic levels of acetaldehyde, especially in Asia.
- A second hypothesis proposes that higher concentrations of acetaldehyde may help to protect against endemic diseases or parasites in Asia.
- Furthermore, the ethnic distribution of ADH and ALDH variants appears to correlate with cultural differences which may be cause or consequence of enzymatic changes. Thus, the ready availability of safe drinking water is a very recent phenomenon due to technical innovation. To avoid pathogens and disease, our ancestors either boiled water and drank tea (Eastern World) or produced and consumed alcohol (Western World). In Asia this cultural preference would have allowed a positive selection for potential advantageous properties of the ADH1B*2 and ALDH2*2 variants whereas in Western cultures selection for efficient alcohol detoxification would have been the stronger selection criterion.












![By Leonard Darwin (Woodall 1884) [Public domain], via Wikimedia Commons](https://droso4schools.files.wordpress.com/2015/04/charlesdarwin.jpg?w=200&h=261)
Pingback: Why the Fly? | flyfacility
Pingback: Bringing life into biology lessons: using the fruit fly Drosophila as a powerful modern teaching tool | Gedankenexperimente
Pingback: Bringing life into biology lessons: using the fruit fly Drosophila as a powerful modern teaching tool | Pedagoo.org
Pingback: A droso4school CPD event for teachers | droso4schools
Pingback: How to communicate basic research in schools – a case study using Drosophila | Gedankenexperimente
Pingback: Genes to Genomes: a blog from the Genetics Society of America
Pingback: Drunk fruit flies - Axonology